2022 Chemistry
- The sub-atomic particles located in the nucleus of an atom are?
- A. neutron and proton
- B. proton and electron
- C. proton and ions
- D. neutron and electron
2.
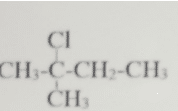
The IUPAC nomenclature of the structure is?
- A. 3-chloro-3-methylbutane
- B. 2,2,-dichloro-3-methylbutane
- C. 3-methylchlorobutane
- D. 2-chloro-2-methylbutane
- SO2+ O2→ 2SO3
In the reaction above, the most suitable catalyst is?
- A. chromium(vi)oxide
- B. iron(iii)oxide
- C. copper(i)oxide
- D. vanadium(v)oxide
- In the preparation of salts, the method employed will depend on the?
- A. composition
- B. dissociating ability
- C. stability to heat
- D. precipitating ability
- The following non-metal form acidic oxides with oxygen except?
- A. phosphorus
- B. sulphur
- C. carbon
- D. chlorine
- How many neutrons are present in atom with mass number and atomic number 37 and 17 respectively?
- A. 18
- B. 20
- C. 37
- D. 17
- The sulphide that is commonly used in coating electric fluorescent tubes is?
- A. iron(ii)Sulphide
- B. tin(ii)sulphide
- C. Zinc Sulphide
- D. lead(iv) Sulphide
- An organic compound which liberate carbon(iv)oxide from trioxocarbonate(iv) solution is likely to be?
- A. C2H5OH
- B. C3H4
- C. C6H6
- D. CH3COOH
- Addition of sodium chloride to water to form a solution would lead to?
- A. increase in freezing point and increase the boiling point
- B. increase in freezing point and decrease the boiling point
- C. decrease in freezing point and decrease the boiling point
- D. decrease in freezing point and increase the boiling point
- A chemical widely used as a fertilizer is?
- A. galena
- B. bauxite
- C. emerald
- D. nitrochalk
- On the basis of the electrochemical series, which of these ions will show the greater tendency to be discharged at the cathode in an electrolytic cell
- A. cu2+
- B. sn2+
- C. fe2+
- D. zn2+
- Addition of charcoal to the filter bed of sand during water treatment for township supply is to?
- A. prevent goiter
- B. prevent tooth decay
- C. remove odour
- D. kill germs
- Using the metal activity series, the metal that can liberate hydrogen gas from steam is?
- A. iron
- B. copper
- C. tin
- D. lead
- An organic compound with fishy smell is likely to have a general formula?
- A. RCONHR1
- A. RCONH2
- C. RNH2
- D. RCOR1
- N2O4⇔ 2NO2 (Δ = -ve)
From the reaction above, which of these conditions would produce the highest equilibrium yield for N2O4?
- A. Low temperature and high pressure
- B. Low temperature and low pressure
- C. high temperature and low pressure
- D. high temperature and high pressure
- Zn + 2HCL → ZnCl2+ H2
What happens to zinc in the above reaction?
- A. oxidized
- B. a reactant
- C. reduced
- D. a metal
- An organic functional group which can likely decolorize ammoniacal silver nitrate is?
- A. alkene
- B. alkane
- C. alkyne
- D. alkanol
- A colored gas that is known to be poisonous and can readily damage the mucous lining of the lungs is?
- A. hydrogen sulphide
- B. carbon(ii)oxide
- C. chlorine
- D. sulphur(iv)oxide
- In order to electroplate spoon with silver, the arrangement of the electrolytic cell is?
- A. the anode is a silver rod and the cathode is the spoon
- B. the anode is the spoon and the cathode is a silver rod
- C. the electrolyte is silver trioxonitrate(v)( solution and the cathode is a silver rod.
- D. the electrolyte is silver trioxonitrate(v) solution and the anode is the spoon
- An organic compound which decolorizes bromine water is likely to be?
- A. C3H8
- B. C2H6
- C. C4H10
- D. C2H4
- Crude petroleum is converted to useful products by the process of?
- A. fractional crystallization
- B. fractional distillation
- C. filtration
- D. chromatography
- An organic compound contains 69% carbon, 15.3% hydrogen and 30.7% oxygen. Calculate the the empirical formula [C=12, H = 1, O = 16]
- A. C4H12O
- B. C3H9O
- C. C4H9O
- D. C3H8O
- 2H2+ O2→ 2H2O
From the equation above, calculate the volume of unreacted oxygen gas if a mixture of 50cm3 of hydroden and 75cm3 of oxygen are involved
- A. 85cm3
- B. 50cm3
- C. 125cm3
- D. 55cm3
- In which of the following will hydrogen form ionic compound?
- A. HCL
- B. NaH
- C. NH3
- D. CH4
- If the volume of a given mass of a gas at 0ºc is 29.5cm33. What will be the volume of the gas at 15ºc, given that the pressure remains constant.
- A. 31.6
- B. 62.2
- C. 32.7
- D. 31.1
- The reactions below represent neutralization reaction, in which of them is the value of ΔH highest?
- A. CH3CH2COOH + KOH → CH3CH2COOK + H2O
- B. NH4OH + HCL → NH4 + H2O
- C. NaOH + HCL → NaCL + H2O
- D. CH3COOH + NaOH → CH3COONa + H2O
- The pollutant usually presents in a city which generates its electricity from coal?
- A. fog
- B. carbon(ii)oxide
- C. smog
- D. sulphur(iv)oxide
- The dehydration of CH3CH2CH2CH2OH will give?
- A. HC≡CCH2CH3
- B. CH2≡CH2CH3
- C. CH3C≡CCH3
- D. CH3CH=CHCH3
- When heat is absorbed during a chemical reaction, the reaction is said to be
- A. thermodynamic
- B. exothermic
- C. isothermal
- D. endothermic
- Which of the following best represent solid gas mixture?
- A. milk
- B. kerosene
- C. soil
- D. smoke
- H++ OH−→ H2O
The equation above illustrates
- A. precipitation
- B. hydration
- C. hydrolysis
- D. neutralization
- Electrons enter into orbitals in order of increasing energy as exemplified by?
- A. 1S22S22PX22py22pz03s0
- B. 1S22S22PX22py12pz13s0
- C. 1S22S22PX12py12pz13s1
- D. 1S22S22PX22py22pz03s1
- Wrought iron is obtained by heating cast iron in a furnace with?
- A. magnetite
- B. haematite
- C. carbon(ii)oxide
- D. calcium trioxosilicate(iv)
- In the extraction of iron, hot air is introduced into the blast furnace through?
- A. valves
- B. open-hearths
- C. arcs
- D. tuyeres
- How many bonding pair are present in carbon(iv)oxide?
- A. 4
- B. 3
- C. 2
- D. 1
- 2-methylprop-1-ene is a structural isomer of?
- A. But-1-yne
- B. 2-methyl But-1-ene
- C. 3-methyl prop-1-yne
- D. But-2-ene
- Alkanes are used mainly?
- A. in the textile industry
- B. in the hydrogenation of oils
- C. as domestic and industrial fuels
- D. as fine chemicals
- What volume of (dm3) of water will be added to 10dm3of 2.0 mol/dm3HCL acid solution to give a final solution of 0.5 mol/dm3?
- A. 30
- B. 40
- C. 20
- D. 50
39
GAS | CO2 | N2 | O2 |
% BY VOLUME | 4 | 72 | 24 |
The above table shows the compositions of the atmosphere of planet X. Which of these gases are present in higher percentages on earth?
- A. CO2 and O2
- B. N2 and CO2
- C. CO2, O2 and N2
- D. O2 and N2
- Hard water is water with high concentrations of dissolved ions, in particular calcium and
- A. magnesium ions
- B. nitrogen ions
- C. phosporus ions
- D. helium ions

